Product Overview
Background
Oxidative stress has been implicated in a number of diseases such as atherosclerosis1, chronic inflammatory disease2, chronic renal failure3, and cancer4. It is a condition where an imbalance exists between the production of reactive oxidizing species and the body’s ability to neutralize these intermediates, resulting in cellular damage. The body has designed several physiological responses to oxidative stress including counterbalances5 such as enzymes and variously functionalized molecules (see examples below) that effectively neutralize these damaging species. These antioxidants can be either water or lipid soluble, and are localized transiently throughout various tissues, cells and cell types.
Given the multiplicity of antioxidant pathways, their centrality in the prevention of oxidant stress, and the influences of lifestyle and nutritional supplements on an individual’s antioxidant capacity, it is important to be able to quantitatively measure the total antioxidant capacity or antioxidant power with biological specimens6-11.
Assay Principles
The reduction potential of the sample or standard effectively converts Cu+2 to Cu+1, thus changing the ion’s absorption characteristics. This reduced form of copper will selectively form a stable 2:1 complex with the chromogenic reagent with an absorption maximum at ca. 450 nm12-16. A known concentration of uric acid is used to create a calibration curve, with the data being expressed as mM uric acid equivalents or in μM copper reducing equivalents.
Typical Standard Curve
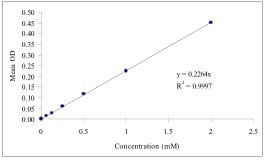
References
1. Brook, R.; (2008) Clin. Sci. (Lond). 115:175-187
2. Cerutti, P. and Trump, B.; (1991) Cancer Cell 3:1-7
3. Cottone, S., et al.; (2008) J. Nephrol. 21:175-179
4. Trachootham, D., et al.; (2008) Antioxid. Redox Signal. 10:1343-1374
5. Frei, B., et al.; (1992) Molecular Biology of Free Radical Scavenging System 23-45
6. Ciencewicki, J., et al.; (2008) J. Allergy Clin. Immunol. 122:456-468
7. Kieth, M., et al.; (1998) J. Am. Coll. Cardio. 31:1352-1362
8. Van-Zoeren-Grobben, et al.; (1997) Acta Pediatrics 86:1356-1362
9. Allard, J.P., et al.; (1998) Am. J. Clin. Nutr. 67:143-147
10. Santini S.A., et al.; (1997) Diabetes 46:1853-1858
11. Fatokun, A., Stone, T. and Smith, R.; (2008) Front. Biosci. 13:3288-3311
12. Apak, R., et al.; (2004) J Agric Food Chem 52:7970-7981
13. Apak, R., et al.; (2005) Free Radic Res 39:949-961
14. Apak, R., et al.; (2007) Molecules 12:1496-1547
15. Apak, R., et al.; (2008) Microchim Acta 160:413-419
16. Apak, R., et al.; (2008) Anal. Chim. Acta 630:28-39
