 Critical tools for the development of therapeutic human antibodies
Critical tools for the development of therapeutic human antibodies
A major emerging class of therapeutic agents are recombinant human monoclonal antibodies. In 2020, five of the top ten highest revenue prescription drugs were therapeutic human monoclonal antibodies (1), and in 2021 the FDA approved the 100th antibody drug. New antibody drugs are being approved for a wide range of applications including the first anti-amyloid antibody for Alzheimer’s disease, a TSLP-targeted antibody for severe asthma (2) and a bispecific antibody for eye diseases (3). Over 1,000 antibodies are currently in clinical development (1). There are a growing range of variations including toxin- antibody conjugates, antibody-drug conjugates (ADCs), bispecific antibodies, Fc- or glyco-engineered antibodies, antibody fragments, and oligonucleotide-coupled antibodies, Accordingly, recombinant human monoclonal antibodies have become a major focus of new drug discovery in the Pharmaceutical and Biotech industries, with IgG1 and IgG4 as the preferred engineered isotypes (4)
Human therapeutic antibody drug candidate molecules must be tested in non-human primate species to assess their pharmacokinetic profile and potential toxicity (5). Primate and human antibodies are highly homologous, making it difficult to distinguish the human antibody drug candidate from the endogenous primate antibodies in a serum or plasma sample by traditional human IgG-specific immunoassays.
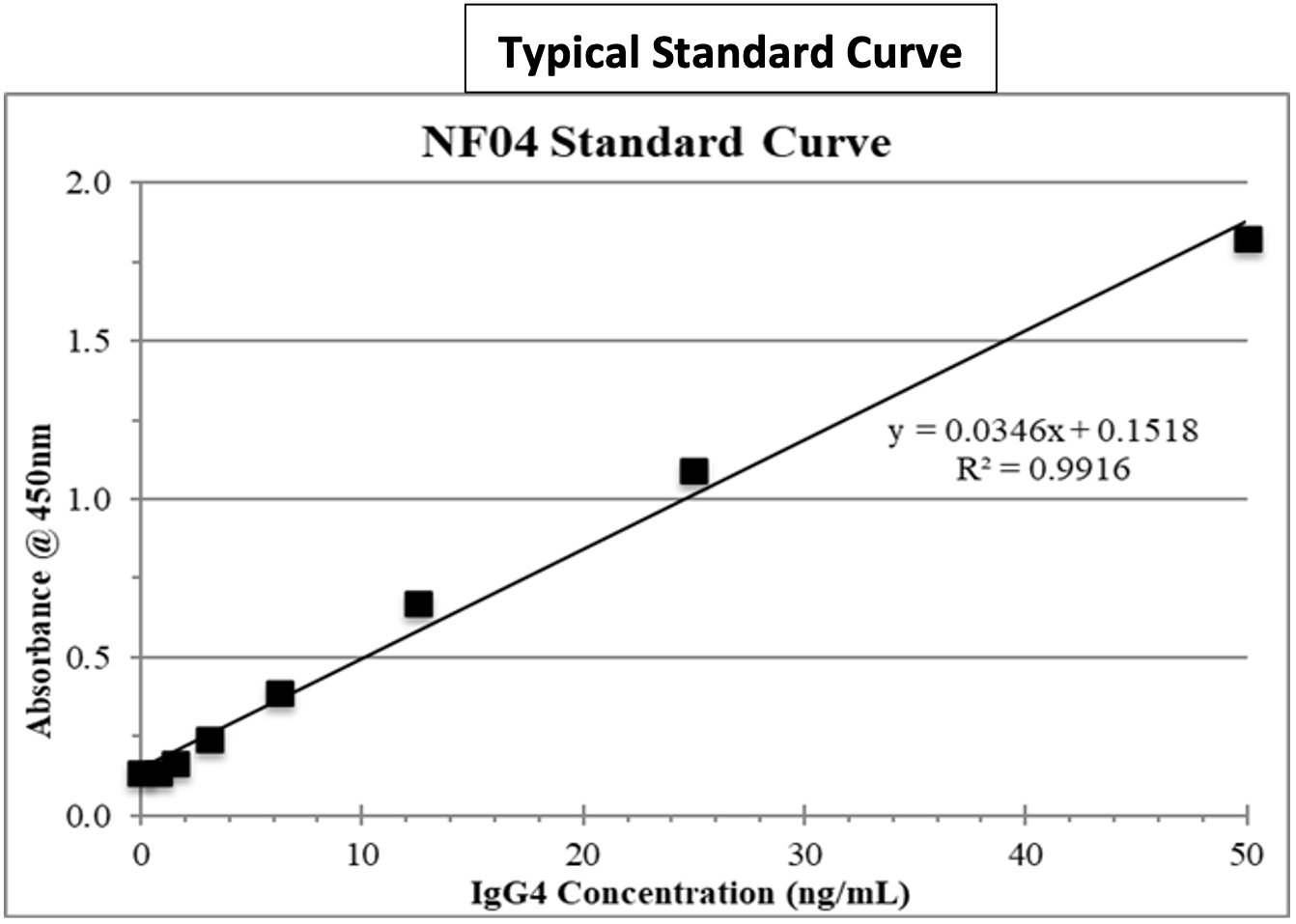
Oxford’s new Recombinant (Therapeutic) Human IgG sandwich ELISA kits can be used to accurately quantify human IgG1 or IgG4 in the serum or plasma of rhesus or cynomolgus monkeys without cross reacting with antibodies produced in those species. These same ELISA kits can also be used to quantify human IgG1 or IgG4 antibodies in the plasma of other preclinical test species (mouse, rat, rabbit, guinea pig, etc.). A recombinant human antibody [IgG1 kappa or IgG4 kappa allotype] is supplied as an internal standard. Alternatively, a specific drug candidate IgG1 or IgG4, respectfully, can be used as a standard in the assay.
Typical Standard Curve
Additional potential applications of the human IgG4 immunoassay may be the quantification of IgG4 antibodies in celiac patients, for which the degree of damage to the intestinal mucosa correlates with serum IgG4 levels (6), and in the diagnosis of IgG4-related disease (IgG4-RD) — a newly recognized systemic chronic fibroinflammatory disease (7).
- Buntz, B. 50 of 2020's best-selling pharmaceuticals. Drug Discovery and Development. May 14, 2021. https://www.drugdiscoverytrends.com/50-of-2020s-best-selling-pharmaceuticals/
- Mullard, A. Nature drug discovery, 2021 FDA approvals. Nature Reviews Drug Discovery 21, 83-88 (2022).
- Mullard A, FDA approves bispecific antibody for two eye diseases. Nat Rev Drug Discov Nature Reviews Drug Discovery February, 2022. DOI: 10.1038/d41573-022-00032-2
- Salfeld, J. selection in antibody engineering. Nat Biotechnol 25, 1369–1372 (2007).
- Stubenrauch K, Wessels U, Lenz H.; Evaluation of an immunoassay for human-specific quantitation of therapeutic antibodies in serum samples from non-human primates. J Pharm Biomed Anal.; 49(4):1003-8 (2009).
- Demircia,H., Polata, Z., Ozturka, K., Kekillic, M. Kantarcioglua, M., Sahiner, F., Uygun, A., and Sait Bagci, S. The degree of mucosal damage to the small intestine and serum immunoglobulin G4 levels correlate with celiac disease Eur/ J. Gastro & Hepatol 27:781–784 (2015).
- Liu, J., Yin, W., Westerberg, L. S., Lee, P., Gong, Q., Chen, Y., Dong, L., & Liu, C. Immune Dysregulation in IgG4-Related Disease. Frontiers in immunology, 12, 738540. (2021) https://doi.org/10.3389/fimmu.2021.738540